Learning Outcomes
i. Define the active site of an enzyme and its significance in enzyme function.
ii. Identify the different types of cofactors and their roles in enzyme activity.
iii. Understand the concept of enzyme specificity and how it is related to the active site.
iv. Explain the impact of cofactor deficiency on enzyme function and human health.
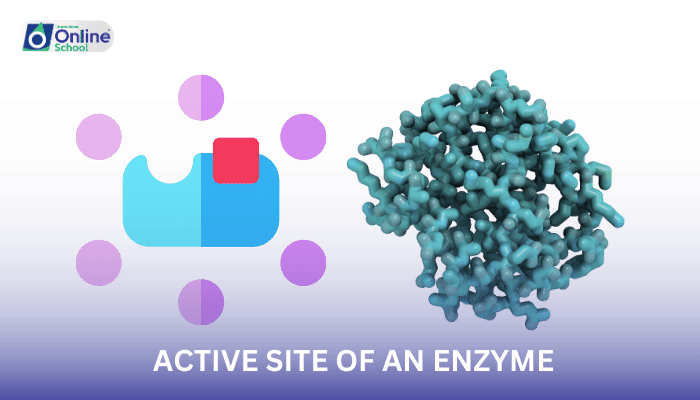
i. The Active Site: The Enzyme's Molecular Spotlight:
Within the intricate structure of an enzyme, a specialized region known as the active site plays a pivotal role in catalyzing chemical reactions. This molecular spotlight, a precisely arranged cluster of amino acid residues, acts as a docking station for the substrate, the molecule upon which the enzyme exerts its catalytic power.
ii. The Delicate Dance of Substrate and Enzyme: A Perfect Fit:
The active site is meticulously sculpted to accommodate the substrate with remarkable specificity, akin to a lock and key mechanism. This precise fit ensures that only the correct substrate can bind to the active site, preventing unwanted reactions and maintaining cellular homeostasis.
iii. Cofactors: The Essential Sidekicks:
While amino acid residues within the active site provide the structural framework for substrate binding, certain enzymes require additional partners to perform their catalytic magic. These non-protein molecules, known as cofactors, act as essential sidekicks, playing crucial roles in enzyme activity.
iv. Inorganic Ions: The Elemental Helpers: Inorganic ions, such as zinc, iron, and magnesium, can act as cofactors, providing stability to the active site or directly participating in the chemical reaction catalyzed by the enzyme.
v. Prosthetic Groups: The Permanently Attached Cofactors: Prosthetic groups are tightly bound organic molecules that are covalently attached to the enzyme's polypeptide chain. These cofactors are integral components of the active site, directly involved in the reaction mechanism.
vi. Coenzymes: The Reusable Assistants:
Coenzymes, also known as organic cofactors, are organic molecules that are not permanently bound to the enzyme but are essential for its activity. These versatile cofactors can shuttle between different enzymes, facilitating a variety of biochemical reactions.
vii. Enzyme Specificity: A Consequence of the Active Site:
The exquisite structure of the active site, with its unique arrangement of amino acid residues and cofactors, is the foundation for enzyme specificity. This remarkable ability of enzymes to recognize and bind only to specific substrates ensures the precision and control of cellular processes, preventing unwanted side reactions.
viii. The Impact of Cofactor Deficiency: A Delicate Balance Disrupted:
A deficiency in cofactors can have profound consequences for enzyme function and human health. For instance, a lack of iodine in the diet can lead to hypothyroidism, a condition characterized by impaired thyroid hormone production due to the requirement of iodine as a cofactor for thyroid peroxidase, an enzyme involved in thyroid hormone synthesis.
The active site, with its intricate architecture and diverse components, serves as the heart of an enzyme's catalytic prowess. The interplay between amino acid residues, cofactors, and the substrate within the active site enables enzymes to orchestrate the symphony of biochemical reactions that sustain life. Understanding the structure, function, and regulation of active sites is not only fundamental to biology but also holds immense potential for the development of new drugs, therapies, and diagnostic tools.